An interview with Dr. Pu-Ting Dong on her award-winning publication
By Jessica Weinstein
ADA Forsyth scientists have spent decades investigating every order, genus, and species of bacteria living in the human oral cavity. Their work accounts for many firsts in this area of research, and they won’t be slowing down any time soon. While some of our world-class microbiologists continue to curate their bespoke taxonomical catalogue of the human oral microbiome – could 100% species identification be in sight? – others are drilling down further into how and why some of these microscopic residents of the oral cavity wield such influence in the state of our oral and overall health.
A newly published study from ADA Forsyth rising star Dr. Pu-Ting Dong, in collaboration with her advisor Dr. Xuesong He, illuminates an interaction between two strains of oral bacteria that produces an uncommon result. This project was selected for a QPC Lasers Young Investigator Best Paper Award by the Chairs of the Conference “Photonic Diagnosis, Monitoring, Prevention, and Treatment of Infections and Inflammatory Diseases” at the SPIE Photonics West conference.
Bacterial family history
“Our lab is the first group to domesticate Saccharibacteria by culturing the first Saccharibacteria strain from human saliva” Dr. Dong explained, recalling the seminal paper on Saccharibacteria (also known as “TM7”) published by ADA Forsyth Drs. Xuesong He and Wenyuan Shi in 2015, before their lab’s move from Los Angeles to Cambridge. Historically considered uncultivable “dark matter” due to some elusive characteristics, Drs. He and Shi successfully cultivated a strain of TM7x and, in doing so, discovered why it had previously been so difficult to culture, as explained by Dr. Dong: “[Saccharibacteria] cannot live on its own. It has to rely on a host bacteria for its metabolism and growth and also, you know, reproduction…our lab is the first to uncover this pair.”
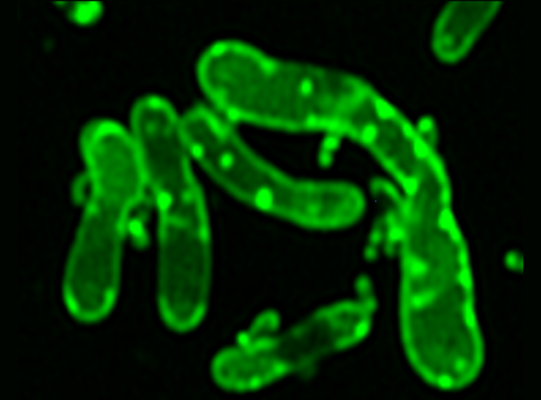
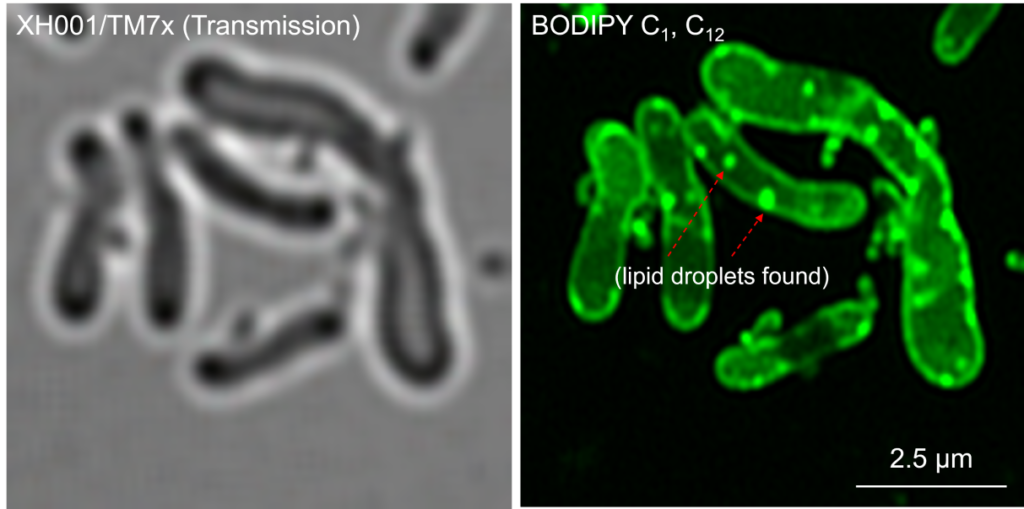
Now with the key to Saccharibacteria’s growth and reproducibility, Dr. Dong has unlocked a new door to a series of events not previously observed by scientists: “This is the first paper to show the intracellular lipid droplet in oral bacteria.”
Something lipid this way comes
As a Postdoctoral Fellow in the He lab, Dr. Dong has the privilege of learning from some of the most valuable players in oral health research. As it turns out, Dr. Dong is also a great teacher herself. “I can give you a metaphor” – she offered to help bring this discovery into context – “it’s like the fat in our body. Basically, the bacteria will have fats in their body, maybe for energy storage purposes or other functions. Our data show that the lipid droplets actually can protect the host bacteria under a stress environment, to promote survival under stress conditions. It’s very similar to us, right? We have fat storage and energy storage, and we can endure a longer time if, for example, an earthquake comes…you can envision it in that way.”
Our data show that the lipid droplets actually can protect the host bacteria under a stress environment, to promote survival under stress conditions…. With this accumulation of fat, they can survive the winter.
Dr. Pu-Ting Dong
Dr. Dong demonstrated her findings with striking images she produced through super-resolution fluorescence microscopy, which no doubt caught the attention of the award selection committee. “As you can see in the beautiful staining, this green actually labels a cell membrane and everything that is hydrophobic…Saccharibacteria is very small, you can see the dots, and when the dots bind with the host, you can see a lot of fats showing up…so this is our first discovery to show this intracellular fat accumulation inside bacteria and in the host cells.” Through diligent re-testing, culturing, staining, and imaging, Dr. Dong and her team were able to independently verify what she describes as “drastic accumulation of intracellular fats” in a coculture system between Saccharibacteria and its host, a phenomenon not commonly observed in any bacteria, and practically unheard-of in oral bacteria.
Having successfully demonstrated the conditions under which these rare lipid droplets form in the oral bacteria-host relationship, Dr. Dong, a trained chemist, infused her expertise into this microbiological study: “I really wanted to know what the chemicals are inside of the fats…Let me show you.” Our interdisciplinary science lesson continued with a primer on Raman spectroscopy: “Our fingerprints can represent us regarding our identity. On this Raman spectrum, it’s just like the fingerprint of the biological system. They are reflecting what kind of metabolites or what kind of chemicals…a global manifestation of the molecules inside the cell.” She employed this technique to conduct a thorough investigation of bacteria accumulated with fat droplets: “this peak is representing the chemical bond vibration…different molecules have different chemical bonds; therefore, their vibrational frequency is different.” By reading the vibrational peaks from different molecules, Dr. Dong was able to create a comprehensive breakdown of nucleic acids, saturated lipids, and other components of the fat droplets.
Having determined what these droplets are and when they occur, there was one question left to explore: why?
What doesn’t kill you makes you stronger
By isolating the host cells and subjecting them to stress conditions, Dr. Dong and her team confirmed that the appearance of the lipid droplets is actually a stress response. Does that mean the presence of Saccharibacteria is a source of stress to its host? “Just like a baby sometimes can cause stress in the mother’s cells, it’s similar here” Dr. Dong explained. “The little bacterium, they cannot survive by themselves. They have to live on the host, something like a parasite.” The experiment continued with a closer look at what, if any, are the benefits of this stress response. “We stressed the host cell first to let them produce lipid droplets, then we did a very interesting experiment. We put this normal one without stress and also the one with the stress in a starving condition for up to three days…The cell will die without nutrition, right? But…if you give them a pre-stress, and then starve them, they can live better! Basically, with this accumulation of fats, they can survive the winter.” She laughed, reflecting on the metaphor. “Sometimes stress is good for us…it goes true for bacteria as well. In our case, it seems Saccharibacteria confer a growth advantage to their host bacteria by inducing the production of intracellular lipid droplets.”
Plenty of dark matter still confounds scientists’ understanding of Saccharibacteria. Dr. Dong says of the future, “a lot of things remain unknown, but we’re here and we contribute a window or a path to understand how they interact with their host bacteria through the intracellular droplets, and how that may impact the interaction between bacteria and the human host.” The types of oral bacteria studied by Dr. Dong and her team are known to be associated with many serious ailments from periodontal disease to Alzheimer’s and some systemic cancers, posing a particular threat to immunocompromised patients, she explains. She hopes that with continued research and discovery, “we may contribute to the development of novel preventative and/or therapeutic tools to keep these bacteria in check and help people stay healthy.”