Scientists have identified many types of bacteria in the mouth, but many problems remain in understanding how they work with one another.
One of the problems is that microbes assemble themselves into densely packed multi-species biofilms. Their density and complexity pose acute difficulties for visualizing individual cells and analyzing their interactions at single-cell level.
ADA Forsyth scientists have developed a new imaging approach that makes it possible to analyze the spatial connections between bacteria, including the strength of adhesive forces that hold them together. Adhesion is of fundamental importance in microbial biofilms subject to flow, such as those in the mouth. Because of salivary flow, any oral microbe that does not adhere to an oral surface such as teeth, tongue or gums, either directly or indirectly via interacting with other adhered microbes, will be flushed into the gastrointestinal tract.
The ADA Forsyth scientists detail their findings in a new paper published in the journal Proceedings of the National Academy of Science. While traditional expansion microscopy techniques are used as resolution-enhancing methods, the ADA Forsyth scientists take a different approach; they use an unconventional application of expansion microscopy which enables the ‘decrowding’ of individual bacterial cells within a multispecies biofilm.
The technique relies on a swellable gel that expands the space between bacterial cells but does not expand the space within the cells. The swelling of the gel creates a ‘tug-of-war’ competition between expansion forces of the gel and adhesive forces between the microbes. Weakly adherent cells will be pulled apart while strongly adherent cells will remain together. In this way, the relative strength of microbe-microbe interactions within microbial biofilms can be determined at the single-cell level.
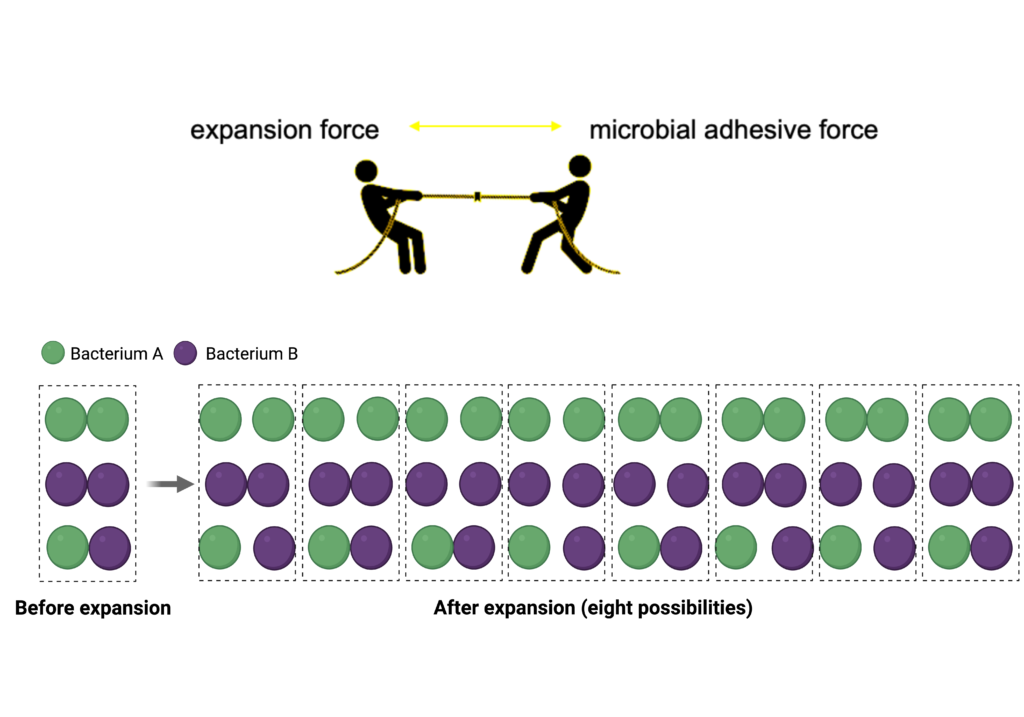
The ADA Forsyth scientists applied the new approach to analyze model microbial communities, specifically Fusobacterium nucleatum-Streptococcus and Schaalia-TM7 interactions. They found that Fusobacterium nucleatum bound more strongly to Streptococcus sanguinis than to Streptococcus mutans. This may be important because Streptococcus mutans is commonly associated with dental cavities.
“Before, we could identify bacterial species in the oral microbiome, but now we can study the way the microbes in these biofilm communities interact,” said Pu-Ting Dong, PhD, lead researcher and the co-corresponding author on the paper. “We can really pinpoint at a single-cell level how strong Bacterium A binds with Bacterium B or Bacterium B with Bacterium C.”
The ability to analyze adhesive interactions between bacteria is groundbreaking, enabling new discoveries in microbiology. The effort combines the deep knowledge and experience of senior members of the ADA Forsyth microbiology department, including AFI Chief Executive Officer, Wenyuan Shi, PhD; Xuesong He, DDS, PhD, a pioneer in microbiology; and Gary Borisy, PhD, a renowned scientist who first introduced CLASI-FISH imaging, the technique that allows scientists to analyze spatial communities in the oral microbiome.
“This interdisciplinary project allowed us to pair what we know about microbial communities with an unconventional way of visualizing their interactions,” Dr. Borisy said. “We can apply this discovery to improve our understanding of the relationships between bacteria. Dr. He was the first to cultivate TM7, a ubiquitous group of ultrasmall episymbiotic bacteria residing within the human oral cavity yet with understudied biology, and now we have used the new imaging technique to determine the strength of TM7’s interaction with its host cell, Schaalia odontolytica.”
Traditionally, individual bacteria species have been recognized as causes of disease in the oral cavity. Oral microbiologists now understand that individual pathogens are not the only cause of disease. Rather, the whole community of microbes interact with each other and the human host in complex ways, sometimes resulting in disease.
Funding:
This work was funded by: National Institute of Dental and Craniofacial Research of the NIH under awards K99 DE033794 (to P.-T.D.), DE022586 (to J.L.M.W and G.G.B.), and DE023810 and DE 030943 (to X.H.).
Paper cited:
“Adhesive interactions within microbial consortia can be differentiated at the single-cell level through expansion microscopy,” Proceedings of the National Academy of Science. DOI: 10.1073/pnas.2411617121

