Introduction
Dr. Trackman joined the ADA Forsyth Institute in November of 2020 from Boston University Henry M. Goldman School of Dental Medicine, where he has been a faculty member since 1992 and where he served as the Director of Oral Biology Graduate Programs. He is best known for his work in the understanding of the biosynthesis and functions of the lysyl oxidase family of proteins. Lysyl oxidases consist of a family of proteins derived from 5 related genes, which produce multifunctional proteins important in connective tissue biosynthesis. A current area of interest is in mechanisms of diabetic bone disease, in which he has recently provided evidence for dysregulation of osteoblast lysyl oxidase by gastric hormones to be a determining mechanism in this pathology (2). Diabetic bone disease results in direct complications to bone maintenance and healing, and among other problems, creates obstacles to desired clinical outcomes for patients undergoing periodontal and other dental procedures. He has also contributed to the understanding of important roles for lysyl oxidases in oral, breast, and prostate cancers (3-8). Dr. Trackman became interested in cancer research in 1990 after cloning the first known form of lysyl oxidase for the very first time when he served as a junior faculty member at Boston University School of Medicine, Department of Biochemistry (9,10). This enzyme family has tumor inhibitory properties, and also paradoxically independently enhances tissue fibrosis and metastasis. The lysyl oxidase propeptide was thought to be a byproduct with no important function of its own was shown by Dr. Trackman’s lab in 2004 to be responsible for the tumor growth inhibitory properties of lysyl oxidase (5). His lab has confirmed this in animal models of both breast and prostate cancer. In addition, molecular mechanisms by which the observed high levels of lysyl oxidase like-2 promotes oral cancer and metastasis was recently uncovered by his lab (3).
He has collaborated extensively with Dr. Alpdogan Kantarci on important aspects of drug-induced gingival overgrowth in which unique aspects of oral connective tissue and epithelial cells were identified and are likely to contribute to the unique oral tissue specificity of this clinical condition (11,12). This work additionally resulted in identifying potential therapeutic opportunities to address fibrotic forms of gingival overgrowth (13).
In addition to his scientific pursuits, Dr. Trackman enjoys classical music, biking, and adventures in Maine with his wife and their two dogs.
Field(s) of Interest: Connective tissue biosynthesis related to pathologies, lysyl oxidases, diabetic bone disease, oral cancer.
Current Projects
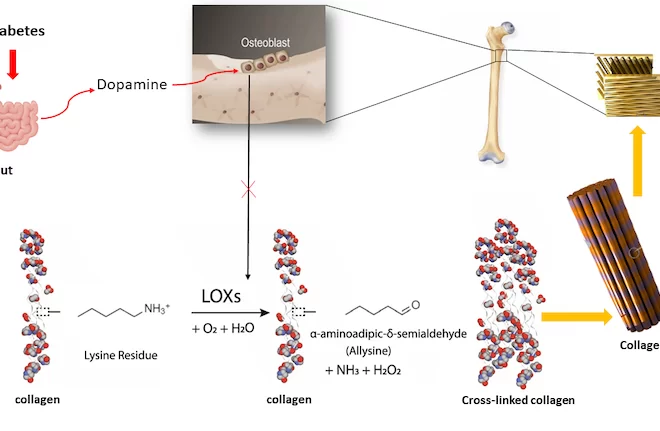
Project Name: Osteoblast Dopamine Receptor Mediates Diabetic Bone Disease
Description: Dopamine is best known as a brain chemical and neurotransmitter that controls mood. However, dopamine is also produced by the gut and directly regulates cells and tissues outside of the brain in the body’s periphery. Here, the hypothesis, based on our initial study (2) is that gut-derived dopamine occurs at abnormally elevated levels in type 1 and type 2 diabetes, and that elevated dopamine levels directly inhibits bone synthetic cells’ (osteoblasts) ability to produce more bone. Our data points to a mechanism in which the ability of another gut hormone known as Glucose-dependent Insulinotropic Polypeptide or GIP to stimulate bone formation is normally balanced by dopamine. When peripheral dopamine levels become too high in diabetes, we propose that bone formation becomes abnormally low. To prove this pathway, we are currently conditionally knocking out dopamine D2 receptor in osteoblasts, and will determine whether mice become resistant to the effects of both type 1 and type 2 diabetes on bone structure and integrity.
Project Name: Therapeutic Opportunities and Mechanisms in Oral Cancer
Description: Lysyl oxidase is best known for its functions in collagen and elastin maturation during the biosynthesis of these important connective proteins. Additional functions have been discovered including the tumor promoting effects of the active enzyme and those of the other four lysyl oxidase paralogues, and the tumor suppressor functions of the lysyl oxidase propeptide, which is released during the biosynthesis of lysyl oxidase (1). We are developing a project for preclinical studies that seeks to take advantage of increased understandings of the mechanisms of both activities to determine the feasibility of some novel therapeutic opportunities presented by these understandings. In addition, we expect that an increased mechanistic understanding will lead to additional potential therapeutic approaches.
Project Name: Mechanism of a Novel Mutation in the Lysyl Oxidase Gene that May Result in Increased Susceptibility to Aortic Aneurysm
Description: We have been made aware of a mutation in the lysyl oxidase gene that appears to predispose affected subjects to aortic dilation and aneurysm. We are investigating the consequence of this mutation on the biosynthesis of lysyl oxidase in vitro, and on the ability of smooth muscle cells to proliferate and differentiate employing induced pluripotent stem cell technology. By analyzing alterations in gene expression of wildtype and normal cells, we hope to learn more about the effect of this mutation on the role of lysyl oxidase in smooth muscle cell differentiation and aortic disease.
References
1. Trackman, P. C. (2016) Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert opinion on therapeutic targets 20, 935-945
2. Daley, E. J., Pajevic, P. D., Roy, S., and Trackman, P. C. (2019) Impaired Gastric Hormone Regulation of Osteoblasts and Lysyl Oxidase Drives Bone Disease in Diabetes Mellitus. JBMR Plus 3, e10212
3. Mahjour, F., Dambal, V., Shrestha, N., Singh, V., Noonan, V., Kantarci, A., and Trackman, P. C. (2019) Mechanism for oral tumor cell lysyl oxidase like-2 in cancer development: synergy with PDGF-AB. Oncogenesis 8, 34
4. Min, C., Kirsch, K. H., Zhao, Y., Jeay, S., Palamakumbura, A. H., Trackman, P. C., and Sonenshein, G. E. (2007) The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer research 67, 1105-1112
5. Palamakumbura, A. H., Jeay, S., Guo, Y., Pischon, N., Sommer, P., Sonenshein, G. E., and Trackman, P. C. (2004) The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. The Journal of biological chemistry 279, 40593-40600
6. Palamakumbura, A. H., Vora, S. R., Nugent, M. A., Kirsch, K. H., Sonenshein, G. E., and Trackman, P. C. (2009) Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene 28, 3390-3400
7. Bais, M. V., Kukuruzinska, M., and Trackman, P. C. (2015) Orthotopic non-metastatic and metastatic oral cancer mouse models. Oral oncology 51, 476-482
8. Bais, M. V., Nugent, M. A., Stephens, D. N., Sume, S. S., Kirsch, K. H., Sonenshein, G. E., and Trackman, P. C. (2012) Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts. PloS one 7, e31188
9. Trackman, P. C., Pratt, A. M., Wolanski, A., Tang, S. S., Offner, G. D., Troxler, R. F., and Kagan, H. M. (1990) Cloning of rat aorta lysyl oxidase cDNA: complete codons and predicted amino acid sequence. Biochemistry 29, 4863-4870
10. Kenyon, K., Contente, S., Trackman, P. C., Tang, J., Kagan, H. M., and Friedman, R. M. (1991) Lysyl oxidase and rrg messenger RNA. Science 253, 802
11. Trackman, P. C., and Kantarci, A. (2004) Connective Tissue Metabolism and Gingival Overgrowth. Critical Reviews in Oral Biology & …
12. Trackman, P. C., and Kantarci, A. (2015) Molecular and clinical aspects of drug-induced gingival overgrowth. Journal of dental research 94, 540-546
13. Assaggaf, M. A., Kantarci, A., Sume, S. S., and Trackman, P. C. (2015) Prevention of phenytoin-induced gingival overgrowth by lovastatin in mice. The American journal of pathology 185, 1588-1599
Publication Listing
- Zhang Y, Liu L, Peymanfar Y, Anderson P, Xian CJ. Roles of MicroRNAs in Osteogenesis or Adipogenesis Differentiation of Bone Marrow Stromal Progenitor Cells. International Journal of Molecular Sciences, 2021.
- Su YW, Fan J, Fan CM, Peymanfar Y, Zhang YL, Xian CJ. Roles of apoptotic chondrocyte-derived CXCL12 in the enhanced chondroclast recruitment following methotrexate and/or dexamethasone treatment. Journal of Cellular Physiology, 2021.
- Fan J, Su YW, Hassanshahi M, Fan C, Peymanfar Y, Piergentili A, Del Bello F, Quaglia W, Xian CJ. β-Catenin signaling is important for osteogenesis and hematopoiesis recovery following methotrexate chemotherapy in rats. Journal of Cellular Physiology, 2020.
- Khabbazi S, Hassanshahi M, Hassanshahi A, Peymanfar Y, Su YW, Xian CJ. Opioids and matrix metalloproteinases: The influence of morphine on MMP-9 production and cancer progression. Naunyn-Schmiedeberg’s Archives of Pharmacology, 2019.
- Hassanshahi M#, Khabbazi S#, Peymanfar Y#, Hassanshahi A, Hosseini khah Z, Su YW, Xian CJ. Critical Limb Ischemia: current and novel therapeutic strategies. Journal of Cellular Physiology, 2018. #Authors contributed equally to this article.
- Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini khah Z, Peymanfar Y, Ghalamkari S, Su YW, Xian CJ. Adipose-derived stem cells for wound healing. Journal of Cellular Physiology, 2018.
- Su YW, Chim SM, Zhou L, Chung R, Ruan CS, Fan C, Foster BK, Prestidge CA, Peymanfar Y, Zhou XF, Xu J, Xian CJ. Osteoblast derived-neurotrophin-3 induces cartilage removal proteases and osteoclast-mediated function at injured growth plate in rats. Bone, 2018.
- Peymanfar Y., Taylor-Robinson, AW. Plasmodium Sexual Stage Parasites Present Distinct Targets for Malaria Transmission-Blocking Vaccine Design. International Journal of Vaccines and Immunization, 2016.
- Vahedi, F., Nazari, N., Arbabi, S, Peymanfar Y. Investigation of DNA integration into reproductive organs following intramuscular injection of DNA in mice. Reports of Biochemistry & Molecular Biology, 2012.
